The early stages of the B-cell system are key for cellular immunity development. The development of functional B cells requires appropriate regulation of dynamic transcription-factor networks. These regulatory networks are responsible for lineage-specific gene expression activation and lineage commitment. Thus, understanding the gene regulatory network (GRN) of this system is essential for studying healthy development and malignant transformations. To this end, in the present study, we aim to uncover the complete chart of the cis-regulatory elements and their dynamic activity over early B-cell commitment in healthy humans.
We generated matched human data for chromatin accessibility (ATAC-Seq) and transcriptome (RNA-Seq) in eight sorted B-cell subpopulations (HSCs, CLPs, pro-B cells, pre-B cells, Immature B cells, and Transitional B cells as well as CD5+ and CD5- Naive B cells) isolated from bone marrow and peripheral blood from the same healthy donors (n=13). These comprehensive profiles of the stepwise progression of B-cell precursors and the combined analysis of both omics allowed us to infer the regulatory elements of B-cell differentiation and the resulting GRNs.
Our data identified 169 TFs, 7,310 genes, and 16,074 OCRs governing the early B-cell differentiation, resulting in a GRN with 377,268 TF-OCR-gene interactions. The inferred GRN included genes and OCRs able to draw the entire regulatory dynamics over differentiation behaving in accordance with established knowledge. As example, we identified genes and TFs related to homeostasis and stemness, such as MEIS1, CD34, and CDH2, and antiapoptotic genes such as BCL2, highly expressed in HSC, CLP, and pro-B cells. We identified surface marker genes and TFs involved in the commitment and differentiation of B cell lineage, including CD79A/B, PAX5, TCF3, EBF1, and FOXO1, to become upregulated in pro-B cells, as well as down-regulation in pre-B cells of genes linked to T cell development such as NOTCH1.
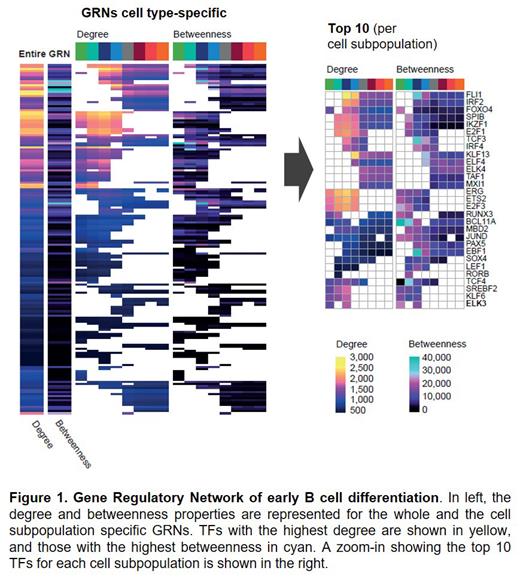
To identify the key regulatory elements along B cell differentiation, we generated a snapshot of the GRN for each cell subpopulation. As a result, the networks' node hub architectures revealed those TFs with relevant transcriptional activity per subpopulation (Figure 1). In the analysis we recovered the primary TFs involved in B cell commitment, differentiation, maintenance, and class-switch recombination: TCF3, BCL11A, IRF8, PAX5, REL, IRF4, EBF1, POU2F2, FOXO1, and LEF1; but also identified ELK3 as a novel TF critical in pro-B cells. Interestingly, this ETS-factor, recently defined as a novel oncogene, has been reported to increase in ETV6-RUNX1+ acute lymphoblastic leukemia cases and other carcinogenic processes. Moreover, it has been associated with pro-B cell expansion.
In a deeper approach, our GRNs allowed the exploration of upstream regulators of specific genes. In the case of ELK3, the TFs ERG, STAT5A/B, E2F3, ETS2, ETV6, SPIB, and POU2F2 were uncovered as putative regulators. As a positive control, we explored the upstream regulation of EBF1 gene, a well-known and key TF of B cell fate. Besides the already described regulators (PAX5, TCF3, FOXO1, RUNX1, etc.), we identified new candidates such as MLXIP.
Finally, we translated our findings into a disease context using public data. Our resource facilitated the identification of triggers for specific B-cell acute lymphoblastic leukemia (B-ALL) subtypes, pinpointing the inflection point of malignant transformation. We found ETV6-RUNX1 fusions significantly enriched in pro-B cells, MEF2D fusions and TCF3-PBX1 mutations in pre-B cells, DUX4 fusions in HSCs, and KMT2A fusions in CLPs. Moreover, our data shed light on the functional implications of B-ALL susceptibility loci. The susceptibility locus rs17481869, associated with ETV6-RUNX1 patients, was identified within a small OCR specific to pro-B cells. The ALL-associated rs4762284 locus was observed in a closed chromatin region, but we could identify two proxies (rs2268508 and rs17736737) in open regions. Furthermore, from the list of SNPs associated with BCP ALL-related traits from the GWAS catalog (NHGRI-EBI), we identified 11 SNPs overlapping some of our OCRs.
In summary, we are presenting the most comprehensive publicly available atlas of early B cell regulation (B-rex; https://brex.shinyapps.io/brex/), and therefore a powerful resource for understanding early B cell differentiation in health and disease.
Disclosures
Paiva:EngMab: Research Funding; Takeda: Honoraria, Research Funding; GSK: Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Roche Glycart AG: Honoraria, Research Funding; Adaptive: Honoraria; Amgen: Honoraria; Gilead: Honoraria; Oncopeptides: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal